Researchers Reveal New Drug To Help Babies With Severe Respiratory Virus
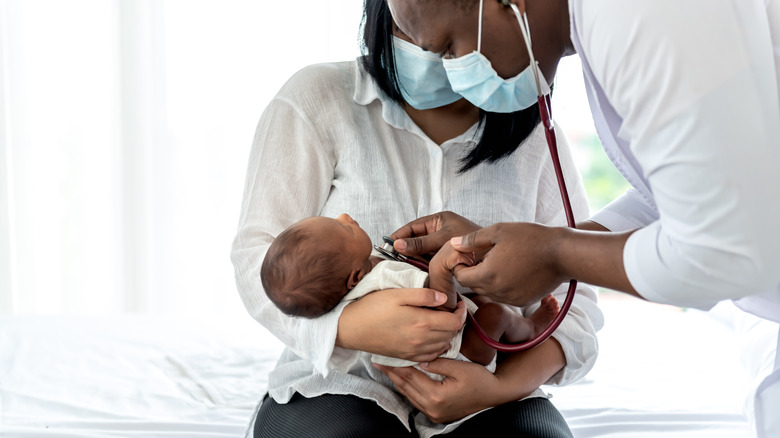
Respiratory syncytial virus, or RSV, is a common respiratory illness that can cause severe symptoms in vulnerable populations, such as infants, the elderly, and those with weakened immune systems (via MedlinePlus). Before now, there's been no preventative medicine or specific treatment for RSV. But after decades of trying, a 2022 study published in the New England Journal of Medicine has found that a new drug may be effective at preventing RSV-related illness in infants.
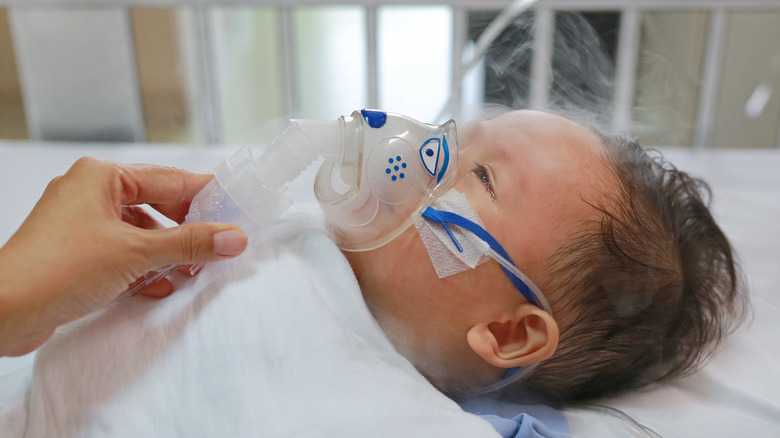
RSV is a common virus that can affect anyone, but it most often occurs in children, according to MedlinePlus. In fact, most children under the age of two will become infected in RSV. It usually presents as nothing more than a common cold, including symptoms like a runny nose, cough, sneezing, fever, and loss of appetite. But for those at high-risk, like very young infants, it can lead to severe lung infections, like bronchitis and pneumonia, often resulting in hospitalization. Researchers have spent years trying to develop a safe vaccine for RSV, without luck — until now.
How a new drug may help protect infects against RSV
Researchers at AstraZeneca have developed a one-time injection, called nirsevimab, that helps to protect against RSV infection in infants (per NBC News). A group of nearly 1,500 infants was studied, each baby assigned to either a placebo or nirsevimab group. They found that the drug was nearly 75% effective at preventing RSV-related lower respiratory tract infections, which are the leading causes for RSV hospitalization in infants.
Despite being a preventative injection, the drug is not classified as a vaccine, or active immunization, since it doesn't cause the body to create antibodies against the virus (via NBC News). Rather, nirsevimab deposits antibodies directly into the bloodstream, which is called passive immunization. This inoculation will eventually wear off, but scientists believe that one injection at the beginning of the RSV season (fall and winter in the U.S.) will be enough to protect infants who are not considered high-risk. For those who are, a second injection may be needed before the next RSV season.
"This protects young infants in that most vulnerable period before their immune system has matured. When they get exposed later in life, they can cope with the disease much better," Tonya Villafana, a senior author on the research, tells NBC News. The study data is expected to be submitted to the Food and Drug Administration in the coming months.