Pfizer's COVID-19 Pill Paxlovid Explained
Back in February of 2020, just weeks before the World Health Organization would declare that the then-novel coronavirus had become a pandemic, Dr. Anthony Fauci told the Washington Post that once a pandemic begins spreading from person to person in multiple countries, it's no longer possible to contain it. Rather, "you can only mitigate it," as he noted. At that time, the U.S. was still in what Dr. Fauci referred to as the "containment phase."
More than two years later, COVID-19 has been diagnosed more than 500 million times, with more than six million deaths recorded (per WHO) – and despite the launch of several vaccines, it turns out the virus is still a moving target. Now well past containment, we are deep into mitigation, with our current efforts focused on preventing severe illness and death to the extent possible. That goes a long way toward explaining the government's Test to Treat initiative, launched in March 2022 (via the U.S. Health and Human Services' Office of Assistant Secretary For Preparedness And Response (ASPR)).
Test to Treat is aimed at getting those most at risk for serious illness immediate access to life-saving treatment upon testing positive, including two oral therapies whose development was funded in part by the Biden-Harris Administration, per an ASPR fact sheet. Paxlovid, developed by Pfizer, is one such therapy. Join us as we explain all that you need to know about Pfizer's pill to treat COVID-19.
What does Paxlovid do, and who is it for?
On December 22, 2021, the USFDA issued an Emergency Use Authorization (EUA) for Pfizer's Paxlovid, a course of pills that have been shown to be highly effective at treating "mild-to-moderate" COVID-19 in certain adults and children ages 12 and over. Paxlovid is available only by prescription, to individuals who test positive for COVID-19 and are considered at high risk for severe COVID-19. Paxlovid is most effective when initiated within the first five days of symptoms, but that does not seem to be a prerequisite for its availability, at least not at this time. That being said, Paxlovid's EUA specifically excludes its use to treat individuals already hospitalized for severe COVID-19, per the American Medical Association.
Paxlovid contains two active ingredients. The first agent prevents the virus from replicating in an affected individual's body (viral replication is a prerequisite for severe COVID-19 illness, per a 2021 study published in the Journal of Infectious Disease). The second helps to boost Paxlovid's efficacy by slowing the metabolic breakdown of the first agent by the body. We'll get into more detail on these two drugs below.
Paxlovid is associated with some side effects and drug interactions. Nevertheless, the FDA has determined that Paxlovid is so effective at inhibiting the progression of COVID-19 to its most severe form, that the potential benefits of Paxlovid "outweigh [its] known and potential risks," per an April 14 letter from the FDA to Pfizer.
How effective is Paxlovid?
In order to issue an EUA for a particular drug, the FDA must determine, among other things, that "based on the total amount of scientific evidence available including data from adequate and well-controlled clinical trials," it is "reasonable to believe" that the drug is effective at doing what it is claimed that it can do and that the "known and potential benefits ... outweigh the known and potential risks (via HHS/ASPR). Accordingly, before granting an EUA with regard to Paxlovid, the FDA had to determine that Paxlovid is effective and safe enough to justify its use — at least until a better drug comes along, or if currently unknown safety issues tip the balance.
In the case of Paxlovid, the FDA has stated that the "primary data supporting" the EUA are from a "randomized, double-blind, placebo-controlled clinical trial studying Paxlovid for the treatment of non-hospitalized symptomatic adults with a laboratory confirmed diagnosis of [COVID-19]," per FDA. The study involved more than 2,000 adults diagnosed with COVID-19 who were at a known risk for progression to severe disease. Half were given Paxlovid; the other half, a placebo. The Paxlovid group had a less-than-1% rate of hospitalization and no deaths, compared with 6% in the placebo group — which translates to Paxlovid being 89% effective at reducing hospitalizations/deaths from COVID-19. Additionally, one of Paxlovid's known benefits is that it can be administered outside a hospital setting and therefore at a lower cost, according to Nature.
How Paxlovid is supposed to be taken
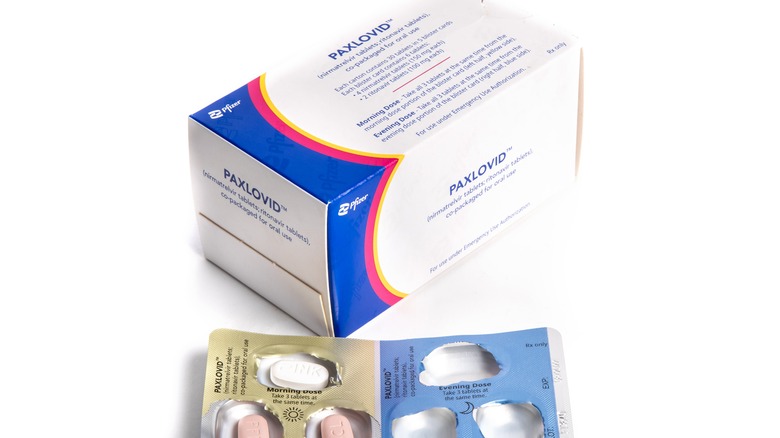
Paxlovid, Pfizer's COVID-19 pill, is made up of two active ingredients. Those are actually two separate medications that are packaged together in three-pill doses, per Yale Medicine. Two of the three pills in each dose consist of a newly developed anti-viral drug (aka protease inhibitor), nirmatrelvir. The other is an existing anti-viral drug known as ritonavir, which was approved by the FDA in 1996. We'll discuss these in more detail below, including what a protease inhibitor is, and why Paxlovid is a combination of two separate protease inhibitors.
A normal course of Paxlovid is the three aforementioned pills, taken twice daily for a total of five days, which ends up being a total of 30 pills. For ease of administration, the two different pills come in handy "dose cards" (not unlike Zithromax, another Pfizer drug), which allow the person who has to take it to "punch out the pills as needed" and thereby more easily keep track of what was taken. Dosage information may be found in more detail in the FDA's Paxlovid Patient Fact Sheet.
What to know about the nirmatrelvir pills in each dose
"All viruses, including SARS-CoV-2, the virus that causes COVID-19, have the same goal: invade our cells, use our cells' internal machinery to make copies of itself, and then release the newly made virus to infect more of our cells," according to Pfizer. Protease inhibitors are drugs that have been developed to interrupt this process by blocking certain enzymes that are required for this process to take place.
Nirmatrelvir is a protease inhibitor that specifically targets the viral protease MPRO, which "plays an essential role in viral replication" in all coronaviruses that are known to be capable of infecting humans, according the National Institutes of Health (NIH). At the same time, it has a "low likelihood of off-target activity," which means that it does not tend to attack anything but the viral protease that it was designed to target (unlike, say, chemotherapy), per this 2022 study published in the New England Journal of Medicine.
In Paxlovid, nirmatrelvir is taken in combination with the drug ritonavir, because ritonavir increases blood concentrations of nirmatrelvir to what the NIH refers to as "target therapeutic range" (per NIH). Accordingly, any side effects associated with nirmatrelvir that the FDA considered in granting its Emergency Use Authorization are those associated with nirmatrelvir taken in combination with ritonavir, as opposed to nirmatrelvir alone.
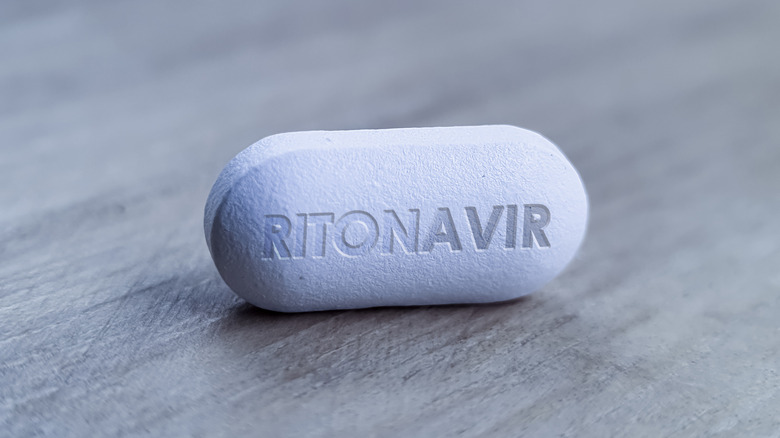
What to know about ritonavir, the other pill in each Paxlovid dose pack
Like nirmatrelvir, ritonavir is also a protease inhibitor. When ritonavir was approved by the FDA in 1996, it was as a single-drug therapy for the treatment of AIDS and the virus that causes it (via StatPearls – NCBI Bookshelf). However, over time, ritonavir has come to be used far more frequently in combination with other anti-viral drugs used in treating viruses that are known for being particularly trenchant, including AIDS, HIV, and hepatitis C (via Precision Vaccinations).
In combination with these other drugs, ritonavir acts as a "booster" to increase the other drugs' effectiveness. The reason for this expansion of ritonavir's uses is that ritonavir has been found not just to slow the replication of viruses in human cells, but also to slow the liver's metabolism of some other drugs (by targeting the enzyme responsible for metabolizing them). This allows the other drugs to remain more highly concentrated in the body and for a longer duration — which is precisely why ritonavir has been included in the Paxlovid drug protocol, per the National Institutes of Health and NPR.
Since ritonavir has been around for as long as it has, it has some known side effects, but those side effects will be discussed below to reflect the low dosage of ritonavir that is actually used in Paxlovid and the fact that the ritonavir is one of two active agents that may cause side effects.
There is a long history behind Pfizer's rapid development of Paxlovid
Just as the rapid development of vaccines against COVID-19 was made possible by the body of vaccine research that already existed prior to the identification of the novel coronavirus that causes COVID-19, Paxlovid stands on the shoulders of a significant body of existing scientific research relating to both of its active ingredients.
First, starting in 2003, and in response to the outbreak of SARS — another illness caused by a coronavirus — Pfizer researchers had been trying to come up with a protease inhibitor that would be capable of stopping coronaviruses (in general) in their tracks by interrupting the process of viral replication (per NPR). Although Pfizer moved on to different challenges after the SARS outbreak was beaten back, the research already in progress took scientists a long way toward coming up with a protease inhibitor that could stop the novel coronavirus that causes COVID-19 from replicating in the human body.
The decision to combine an effective protease inhibitor with a drug that is capable of keeping that protease inhibitor active in the body for as long as safely possible comes out of medical therapies that have been proven safe and successful since the late 1990s, per NPR and WebMD. Ritonavir was a logical choice for this because it has been in use since the 1990s. Once researchers began testing ritonavir-boosted nirmatrelvir, they quickly observed a "90% reduction in hospitalization" and "100% protection against death" (per NPR).
Is Paxlovid really an AIDS drug?
Although some have been referring to Paxlovid as an "HIV drug" (via Twitter), Pfizer's Paxlovid pill regimen was developed specifically to treat COVID-19 (via NPR). That one of Paxlovid's agents was developed to fight the virus that causes AIDS doesn't make Paxlovid an AIDS drug any more than the fact that one of Paxlovid's agents began as a possible drug therapy in connection with the 2003 SARS outbreak makes Paxlovid a "SARS 2003 drug."
The reason Paxlovid makes use of a drug that has saved the lives of many who would otherwise have died from AIDS is that the medical community's war on AIDS yielded a plethora of knowledge about viruses in general, especially about trenchant viruses like HIV. One lesson learned is that anti-viral agents are much more effective at suppressing viral replication when combined with another agent that slows the body's metabolism of some medications. And that is precisely what ritonavir has been found to do over the decades, says Harvard's Dr. Paul Edward Sax (via Reuters). Ritonavir blocks a liver enzyme responsible for rapidly clearing antiviral agents from the blood.
"By blocking [a particular liver] enzyme, the clearance of the [the anti-viral drug] is slowed down, meaning higher levels of the medication can be achieved. This is what ritonavir is doing in Paxlovid," Dr. Sax explains. In sum, nirmatrelvir inhibits replication of the novel coronavirus, and ritonavir keeps nirmatrelvir in the body for an optimal length of time.
What are the common side effects of Paxlovid?
"Paxlovid is usually very well-tolerated," according to Dr. Scott Roberts (via Yale Medicine). Common side effects include an impaired sense of taste, diarrhea, increased blood pressure, and muscle aches, but these are usually mild. The most commonly seen adverse events that were observed in the clinical study that found Paxlovid to be nearly 90% effective at preventing hospitalizations and deaths from COVID-19 were the first two mentioned above, according to the NIH. However, what may be most relevant here is that Paxlovid appears to have been better tolerated than the placebo given to the control group in the study, with fewer experimental subjects having discontinued the study than members of the control group.
All of that being said, Paxlovid should never be prescribed for or taken by people with "uncontrolled or undiagnosed HIV-1 infection," because Paxlovid could lead to HIV-1 drug resistance, according to the FDA and the NIH. Nor should it be prescribed for or taken by anyone with a pre-existing liver disease or condition, including abnormal liver enzyme readings (which could indicate liver inflammation and other issues), because the ritonavir in the regimen directly impacts liver function. Additionally, Paxlovid should not be taken by anyone with severe kidney issues. In people with moderate kidney impairment, Paxlovid may be used, albeit at a reduced dose.
Paxlovid can cause serious drug interactions with commonly used medications and supplements
Paxlovid is associated with "significant and complex drug-drug interactions," according to the NIH. Paxlovid's ritonavir component, which works on the liver to slow metabolism of certain medications to raise their concentrations and keep them in the body for longer, is the primary vector. Accordingly, Paxlovid shouldn't be taken with any drug that is "highly dependent" on the particular enzymes that ritonavir-boosted nirmatrelvir targets and for which elevated concentrations in the blood may cause serious and/or life-threatening adverse effects, per the FDA.
However, Paxlovid should also not be taken in combination with any drug that may increase the effectiveness of those particular enzymes. That may result in the faster breakdown of Paxlovid's active ingredients, which is precisely the opposite effect that one wants when taking nirmatrelvir in combination with ritonavir. Nor should it be taken until any such drugs have left the bloodstream.
A complete list of drugs that should never be taken in combination with Paxlovid are available for you and for your healthcare provider on this fact sheet. Some of the more common ones include statins (for treating high cholesterol), antiplatelet medications given after a heart attack or stent placement, antiarrhythmic medications, epilepsy medications, and opioid pain relievers. In addition, some over-the-counter supplements are contraindicated with Paxlovid, including St. John's wort (per Express Scripts Pharmacy), which is one of the supplements you should probably be steering clear from anyway.
Paxlovid should not be taken by pregnant or lactating women, at least not until further research is conducted
Pregnant women are considered to have an increased risk for developing severe COVID-19, and are therefore encouraged to get vaccinated, per the CDC. Nevertheless, at this point in time, Paxlovid is not approved or emergency-authorized for use in pregnant women or lactating women, according to the CDC. The reason is that Paxlovid is so new that its use in pregnant and lactating women has not yet undergone complete clinical study regarding its safety and efficacy (per Precision Vaccinations).
However, the NIH stated that the ritonavir component of Paxlovid has been used "extensively during pregnancy in people with HIV," suggesting that Paxlovid would present an "acceptable safety profile" for use during pregnancy. The agency also noted that "based on the mechanisms of action for both nirmatrelvir and ritonavir and the available animal data," they would not prohibit a pregnant patient from receiving treatment in the form of ritonavir-boosted nirmatrelvir "if the potential benefits outweighed the potential risks." Thus, depending on the status of any current research, it is possible (if not likely) that Paxlovid will become available for emergency-authorized use in pregnant women in the near future.
Pfizer's Paxlovid may temporarily decrease the effectiveness of certain hormonal birth control protocols
In addition to the drug interactions discussed above, Paxlovid may have the effect of decreasing the efficacy of birth control products that contain the active ingredient ethinyl estradiol, according to the FDA. The reason is that Paxlovid, may have the potential to decrease blood ethinyl estradiol levels, at least for the days on which it is being taken. Accordingly, people on a birth control protocol that contains this hormone may not be protected against pregnancy during the course of treatment, and possibly for as long as Paxlovid remains in one's system. If you're prescribed Paxlovid, you should let your doctor know if you're on such a protocol. In addition, you should also consider using an alternative, non-hormonal contraceptive method for at least the five days during which you are taking Paxlovid, if not longer, depending on your doctor's guidance.
Birth control pills that contain progestin in addition to ethinyl estradiol should not be affected by the taking of Paxlovid, according to Precision Vaccinations. In fact, when Paxlovid is used with such combined hormonal contraceptives, it is possible that progestin levels may increase. Again, it would be advisable to discuss your use of Paxlovid if you're on any form of hormonal birth control.
Paxlovid's role in the Biden Administration's Test To Treat program
In March 2022, the Biden-Harris Administration launched a new program in the fight against COVID-19 called Test to Treat (per the HHS). Under this program, an individual should be able to get tested, receive results, receive a prescription, and have it filled, all from a single location — "One-Stop Test to Treat" sites — of which there are to be hundreds nationwide, including at pharmacies and within health clinics and long-term care facilities.
Of course, seeking testing and treatment from one's own healthcare provider will remain an option, as always. In connection with this program, the Administration has been investing in developing COVID-19 treatments that can be taken in pill form. One of the treatments that has come out of that is Paxlovid.
Express Scripts Pharmacy (ESP) says that Paxlovid will be in short supply initially, with only 65,000 treatment courses having been made available to distribute when the Emergency Use Authorization was issued. However, Pfizer has committed to "supplying 10 million treatment courses by the end of June and a total of 20 million treatment courses in 2022." If you're eligible for Paxlovid and find that it isn't available, there is another emergency use-authorized COVID-19 pill. Made by Merck, it's called molnupiravir. In addition, there is also the option of taking remdesivir, the monoclonal antibody treatment that is available by IV only.
Why Pfizer's Paxlovid pill is more desirable than Merck's Molnupiravir (Lagevrio)
Although two pills are available for preventing a mild to moderate case of COVID-19 from escalating to a hospitalization or death in people at high risk of the same, Paxlovid is more desirable than Merck's mulnopiravir (also sold under the brand name Lagevrio, per Merck). First, molnupiravir was not nearly as successful at reducing hospitalizations and death as Paxlovid. "Of the 709 people who received molnupiravir, 6.8% were hospitalized or died within this time period compared to 9.7% of the 699 people who received a placebo," according to the clinical study which helped the FDA determine in favor of granting molnupiravir its Emergency Use Authorization (via FDA).
By contrast, Paxlovid had a far better rate of success (via GoodRx). Moreover, whereas no patients died from COVID-19 after taking Paxlovid, one did die from COVID-19 despite having been treated with molnupiravir under study conditions. In addition, molnupiravir is not authorized for children under the age of 18. As stated by Dr. Patrizia Cavazzoni, director of the FDA's Center for Drug Evaluation and Research, the EUA for molnupiravir is for an "additional drug" in the fight against COVID-19, but one that is "limited to situations where other FDA-authorized treatments for COVID-19 are inaccessible or are not clinically appropriate" (per FDA).
Paxlovid may be useful in treating long COVID, too
Something we have learned over the last two-plus years is that approximately 30% of all COVID-19 cases may lead to the long-term post-viral syndrome known as "long COVID," according to a 2022 study published in the Journal of General Internal Medicine and WebMD. Healthline describes the most common symptoms of long COVID as "fatigue, cognitive dysfunction, and malaise." Symptoms of long COVID may persist for six months or even longer following initial recovery from an initial bout of COVID-19.
A recent study published in the journal Cell suggested multiple potential factors that may contribute to one's developing long COVID. Since none of these factors (the presence of certain autoantibodies, a high viral load, Type 2 diabetes, and a previous infection with Epstein-Barr virus) are within our immediate control, it may be comforting to know that Paxlovid has shown promise as a therapy for treating long COVID. Granted, that promise is nothing more than a case study of a patient whose long COVID symptoms retreated after taking Paxlovid, per Reuters. That said, some of our most groundbreaking drugs ultimately arose out of an observation made with regard to a single patient, according to University of California, San Francisco medical professor Steven Deeks (via Reuters).
Can COVID-19 come back shortly after finishing a course of Paxlovid?
As game-changing as Paxlovid appears to be, some questions have arisen amid a small handful of anecdotal reports of recurrent COVID-19 after taking Pfizer's COVID-19 pill regimen, according to MedPage Today. Apparently, the NIH has prioritized getting clinical studies underway for the purpose of examining what has been termed a potential "rebound phenomenon," in which people who have been treated with Paxlovid may circumvent severe illness, hospitalization, and death only to experience a return of their original COVID-19 symptoms upon finishing their treatment course (per Bloomberg).
Thus far, there is only one such case that has been documented in medical literature. Nevertheless, in recent weeks, social media users and medical professionals alike have been recounting stories of COVID-19 "rebound." However, these stories are outside of the realm of a clinical study, which means that it is impossible to verify the data, let alone compute their significance. But one question currently concerning many is whether Paxlovid might somehow be to blame. Pfizer believes that is unlikely. The reason is that the so-called rebound phenomenon has also been observed in clinical study subjects who only thought they might have received Paxlovid, but were actually given the placebo that acted as the study's control. In fact, rebound occurred at the same rate in both the experimental and the control groups.