Food Additives That Are Banned In Europe (But Not In The United States)
Food additives are substances added to food products to enhance their safety, preserve freshness, improve taste, manipulate texture, or enhance visual appeal, per the World Health Organization (WHO). They go through rigorous scrutiny for potential adverse effects on human health before they are deemed fit for consumption. Internationally, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) is responsible for evaluating them and ensuring a global standard for safety.
Yet, in the United States, the safety evaluation and authorization of food additives is carried out by the Food and Drug Administration (FDA), according to the Federal Food, Drug, and Cosmetic Act. In contrast, The Scientific Committee on Food (SCF) and the European Food Safety Authority (EFSA) are the entities responsible for conducting safety assessments for all authorized food additives in Europe (per the European Commission). Regardless of geographical location, these agencies meticulously evaluate scientific evidence to determine the safety of additives before granting approval for use in the food market.
Food additives can originate from diverse natural and man-made sources. They are broadly categorized based on their functions. For example, nutritional additives replenish lost nutrients during production. Processing agents assist in food manufacturing or maintaining product consistency, while preservatives extend shelf life by preventing deterioration and inhibiting microbial growth. Lastly, sensory agents, such as colorants and flavorings, compensate for processing losses, and sweeteners provide sweetness with minimal calories (via the Encyclopedia Britannica). Despite the thorough evaluation of these ingredients, responsible entities sometimes have differing views on their safety, leading to discrepancies in permitted additives in different parts of the world.
Titanium dioxide
Titanium dioxide (also known as E171) has been a staple food additive for decades, primarily used to impart a vibrant white color to various foods, ranging from baked goods and sandwich spreads (think mayo) to soups, sauces, and salad dressings. However, recent developments have led to its banning as a food additive by the European Commission, a decision originating from concerns raised by the EFSA (via the European Commission).
While not definitively concluding that E171 poses a confirmed health risk, the EFSA did not rule out the possibility of adverse effects, particularly the potential for genotoxicity, meaning that titanium dioxide might have the capacity to cause DNA damage, raising alarm bells for health authorities in the European Union (EU). One of the key factors contributing to these concerns is the accumulation of titanium dioxide particles in the body following oral ingestion despite their generally low absorption, seeing that there are doubts regarding their long-term effects on animals, consumers, and the environment (via the EFSA). The EU, guided by the precautionary principle, decided to err on the side of caution and banned its use as a food additive in light of the uncertain safety profile.
In contrast, E171 remains a widely used food coloring agent in the United States. In fact, according to New Atlas, it seems to be present in over 11,000 food and beverage products nationwide, as the FDA continues to allow the use of titanium dioxide in food products, provided that it does not exceed 1% by weight of the food (per the FDA).
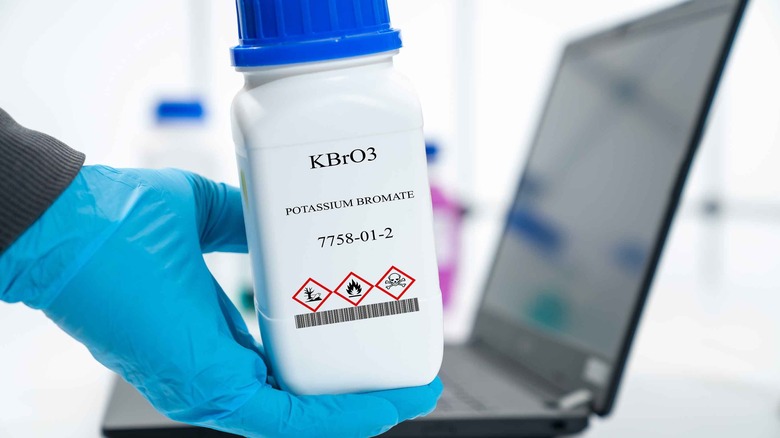
Potassium bromate
Potassium bromate is a chemical compound known for helping bread rise. However, its use has raised significant health concerns due to its potential cancer-inducing properties (via The Guardian). According to a 2021 study published in Science Progress, the International Agency for Research on Cancer has classified potassium bromate as a carcinogen based on studies linking it to kidney and thyroid cancers in rodents. The primary issue with potassium bromate lies in its ability to induce oxidative stress, surpassing the cell's natural antioxidant defenses and causing DNA damage. Potassium bromate is rapidly absorbed, transformed into bromide within body tissues, and partially excreted in urine. Notably, the kidneys are the primary target organs for potassium bromate toxicity, and ingestion can lead to acute poisoning and potentially result in kidney failure.
In response to these health concerns, numerous countries, including Brazil, Canada, the EU, and the United Kingdom, have banned using potassium bromate in food production. However, the United States has not followed suit, and potassium bromate remains legal for use with limited oversight from the FDA, which hasn't reviewed its safety since at least 1973, per the Environmental Working Group (EWG). Regulations in the United States allow only minimal amounts of potassium bromide in bread, specifically less than 75 parts per million*, a quantity the FDA considers to pose a negligible risk of exposure to toxic byproducts (via The Guardian). According to the EWG, the U.S. food industry insists potassium bromate is safe in baked products because it should convert entirely into non-carcinogenic potassium bromide during baking; however, tests in the U.K. have proven otherwise.
Azodicarbonamide
Azodicarbonamide (ADA) is a chemical compound with multiple uses in the food industry. It is approved as a whitening agent in cereal flour and a dough conditioner in bread baking (via the FDA). According to EWG, ADA's introduction into bread making was a significant development, as it allowed flour to achieve kneading maturation without the need for long storage, significantly improving the efficiency of industrial bread production and leading to its approval as a food additive in 1962. However, there are some concerns regarding the health implications of ADA.
The Guardian explains that, during the bread-making process, ADA breaks down into two chemicals: urethane and semicarbazide (SEM). Per the U.S. Department of Health and Human Services, urethane is known to be a human carcinogen, while SEM has been linked to a higher risk of cancer in mice. While the FDA acknowledges the SEM-related findings, it highlights that the amounts of SEM tested in these studies are much higher than what people would typically get from eating bread with ADA. Thus, it maintains that ADA is a safe food additive when used following specified regulations.
In contrast to the U.S. standpoint, the EFSA took a more precautionary perspective and recommended limiting exposure to SEM where possible, leading to the EU's ban on the use of ADA in food production. Similarly, the EWG has also raised awareness about ADA by compiling lists of products containing the chemical. Over the years, the number of products containing ADA has decreased, reflecting some changes in the food industry's practices (via The Guardian).
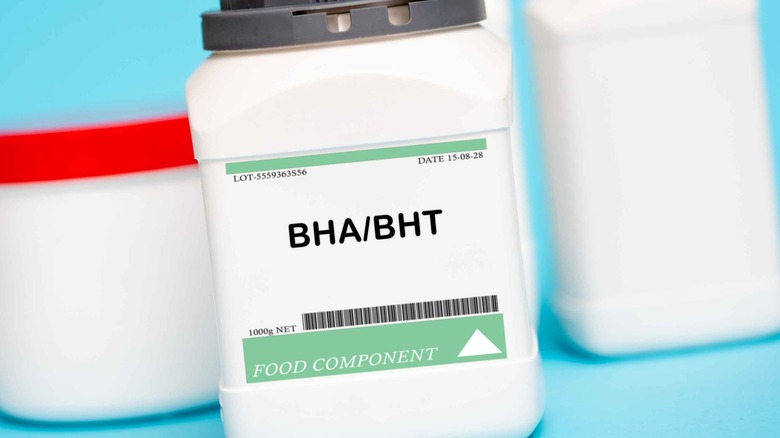
Butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT)
Butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) are food additives used as preservatives and emulsifiers to prevent fats and oils from going bad. In the U.S., these additives can be used in specific foods, including dehydrated potato shreds, dry breakfast cereals, emulsion stabilizers for shortenings, potato flakes and granules, and sweet potato flakes (per the FDA). Though they're not banned in the EU, they have faced strict restrictions in the region due to concerns about their potential health effects and the limited data available on their impact on humans (via The Guardian). While the FDA has permitted the use of these additives in small amounts since the 1950s and contends that they pose no safety concerns at such levels, their long-term effects remain a subject of debate.
For instance, a study published in Environmental International shares that BHA has been linked to tumor promotion and the disruption of the endocrine system (meaning that it may interfere with your hormones and potentially lead to adverse health effects). Meanwhile, the byproducts of BHT have been associated with DNA damage and cancer-promoting properties in animals. Moreover, the EWG has classified BHA and BHT as "of higher concern in food" due to multiple moderate concerns identified by the EFSA, including the possibility of BHT causing liver tumors, birth defects, reproductive and developmental toxicity, thyroid effects, and lung cancer in animals, as well as allergies in humans. Similarly, BHA has been associated with potential cancer risk and hormonal disruption.
Recombinant bovine growth hormone (rBGH)
Recombinant bovine growth hormone (rBGH) is a synthetic hormone designed to increase milk production in dairy cows and has been utilized in the United States since its approval by the FDA in 1993. However, its use is prohibited in the European Union and Canada, among other countries (per the American Cancer Society). According to EWG, the main reasons for rBGH's exclusion in Europe come from concerns about the potential impact on milk's nutritional qualities and animal welfare. European officials note that rBGH treatment might elevate the concentration of insulin-like growth factor (IGF-1) in milk, which has been associated with several chronic diseases in humans, including breast and prostate cancers.
Per the Center for Food Safety, Canadian and European regulators claim that the FDA didn't consider a study demonstrating that the increased IGF-1 in rBGH-treated milk could survive digestion and get absorbed by consumers, a finding that raises questions about the potential health risks associated with consuming dairy products from cows treated with rBGH. Furthermore, the use of rBGH in dairy cows has been linked to an increased incidence of mastitis, a painful udder infection. To treat these infections, dairy farmers often resort to antibiotics, and residues of these antibiotics may find their way into milk and dairy products, adding to the concerns. Still, the FDA claims that rBGH doesn't produce any biological activities in humans and allows its use for 8 out of a cow's 10-month lactation period (per the FDA).
Food dyes
Color additives or food dyes serve various purposes in the food industry, including enhancing natural colors, adding vibrancy to colorless items, making foods more visually appealing, and helping identify flavors in certain products. In the United States, the use of color additives is considered safe when their usage adheres to FDA regulations, despite some evidence hinting at potential allergies in certain children (via the FDA).
While color dyes are not entirely banned across Europe, their regulations and restrictions differ from country to country. In the United Kingdom, for example, several color additives, including Allura Red, Tartrazine, Sunset Yellow, Quinoline Yellow, Ponceau 4R, and Carmoisine, are required to bear a warning label stating that they "may have an adverse effect on activity and attention in children" (via GOV.UK). In contrast, per a study published in Scielo, countries such as Sweden, Switzerland, and Norway have withdrawn Yellow 5 (Tartrazine) due to concerns about its anaphylactic potential, and according to Healthline, Austria and Norway have outright banned Yellow 5, too.
Additionally, a study published in Food Additives & Contaminants: Part A states that the EU does not allow the use of certain synthetic color additives that are approved in the U.S., including Orange B, Citrus Red No. 2, and Green No. 3 (aka Fast Green FCF). Although considered safe by the FDA, Fast Green FCF has been linked to tumor- and mutation-inducing effects in animals and humans (per Drugs.com). Lastly, Red Dye No. 3 has also been banned in the EU due to safety concerns (per NBC News).
Brominated vegetable oil (BVO)
Brominated vegetable oil (BVO) is a food additive created by adding bromine to vegetable oil to stabilize citrus flavoring in beverages, thus preventing it from separating and rising to the surface. In the United States, BVO has been allowed for use with a strict limit not to exceed 15 parts per million (via the FDA). However, concerns about the safety of BVO have prompted the FDA to revise its regulation, proposing the removal of authorization for the use of BVO as a food ingredient based on a study published in 2022, in which high levels of exposure to BVO were linked to potential adverse health effects on the thyroid in rodent studies. Fortunately, companies like Coca-Cola Co. and PepsiCo did not wait for the FDA's rectification and announced the elimination of BVO from their soft drinks in 2014 (via USA Today).
In contrast to the U.S., BVO has been banned as a food additive in Europe since 2008 (via the European Parliament). On that note, the EWG classifies BVO as a substance of "higher concern in food" based on multiple moderate concerns identified in various studies, which comprise brominism in humans (or neurological problems caused by excessive bromine), increases in triglyceride and cholesterol levels, and thyroid effects, reproductive toxicity, and behavioral effects observed in rats.
Propylparaben
Propylparaben is a chemical compound used as an antimicrobial agent in food. Its purpose is to inhibit the growth of microorganisms and extend the shelf life of food products, and in the United States, the FDA allows it to be present at levels not exceeding 0.1% (via the FDA). Yet, despite its widespread use in the U.S., there are some concerns regarding its potential health effects. According to EWG, evidence suggests that propylparaben can disrupt the endocrine system, which plays a critical role in regulating hormones. Chemicals that interfere with hormone signaling have the potential to adversely impact your health, including development, reproduction, and the functioning of the neurological and immune systems.
In contrast to the U.S., which classifies propylparaben as "Generally Recognized As Safe" (or GRAS), the EFSA took a precautionary approach, and in 2004, they reconsidered their thoughts on the level of propylparaben considered safe in food. Consequently, in 2006, they decided to take propylparaben off the list of approved additives for food in the European Union (via EWG). Nonetheless, the FDA has maintained its stance on the safety of this additive in food or food packaging, and according to the Centers for Disease Control and Prevention (CDC), in 2006, the agency aligned with the industry-led Cosmetic Ingredient Review's (CIR) conclusion that parabens, including propylparaben, are still safe.
Ractopamine
According to a study published in The Journal of Pharmacology and Experimental Therapeutics, ractopamine is a livestock feed additive used in the United States on approximately 80% of all beef, swine, and turkey to help animals get heavier, build more muscle, have less fat, eat less food, and produce less waste, a handful of advantages that make it appealing to the livestock industry. However, concerns about its safety have led to its ban in numerous countries, including China, Russia, and some countries within the European Union. Ractopamine belongs to a class of drugs called beta-agonists, initially developed to treat asthma in humans, and its adaptation for animal use was based on its ability to accelerate growth rates in livestock (via Live Science). This transition from human medicine to animal agriculture has raised serious questions about the compound's safety.
On the one hand are the animal welfare concerns. The FDA approved using ractopamine in pigs and later discovered that it caused abnormal heart rates. This prompted the use of warning labels cautioning against its use in breeding swine. However, it did not prevent the additive from being used for that exact purpose. Meanwhile, there are also human health concerns. According to the EFSA, ractopamine and its metabolites in edible tissues can be converted into free ractopamine in the consumer's intestinal tract, raising toxicological and pharmacological concerns for consumers. Therefore, after reevaluating ractopamine in 2009, European food experts decided that there wasn't enough information to prove its safety for people and, thus, in Europe, they don't allow the sale of pork from ractopamine-fed pigs.
Why are they not banned in the United States?
There are clear differences in the approaches to regulating food additives in Europe and the United States. In Europe, safety evaluations of food additives began as early as the '70s, with many additives scrutinized in the '80s and '90s. However, the EFSA plays a key role in regularly reevaluating authorized additives and advising the European Commission on their safety, a process that allows for adjustments in the conditions of use or removal of additives if safety concerns arise (via the European Commission).
In contrast, in the United States, the regulatory landscape for food additives has been shaped by factors such as the FDA's Generally Recognized as Safe (GRAS) loophole, which has allowed some food additives that were used before safety rules were made to stay in foods without being carefully checked. Additionally, the food industry sometimes decides for itself if an additive is safe, which can lead to a lack of good safety data, as pointed out by experts via The Guardian. Furthermore, while legislation such as the 1958 amendment to the Food, Drug, and Cosmetic Act attempted to address potential cancer-promoting additives by banning their approval, it did not apply retroactively to additives already in use at the time, contributing to the presence of certain additives in the market despite safety concerns (via Advisory Board).
Nevertheless, the EWG shares that to address these regulatory gaps and ensure the safety of food additives, Congress is considering the Food Chemical Reassessment Act of 2021, which would strengthen the U.S. food regulatory system and close the regulatory gaps that have allowed certain additives to remain in use without comprehensive safety assessments.
How to avoid potentially risky food additives
You can avoid potentially risky food additives by adopting some simple and mindful practices. For instance, the Children's Hospital of Orange County (CHOC) suggests taking the time to read ingredient labels and compare products when shopping for groceries, seeing that some manufacturers have already started to reduce their use of certain food additives. In addition, opting for whole, unprocessed foods such as fruits, vegetables, whole grains, or homemade foods is an automatic way of controlling the ingredients that go into your meals and ditching unnecessary additives that the food industry only uses to make their foods last longer or to be more visually appealing. Visiting your local farmers' market is another excellent way to minimize food additives.
Yet, if ditching processed foods altogether is not something you feel ready to do at the time, the site explains that going for some simple swaps in your everyday products can also make a big difference. For example, you could use butter instead of margarine or natural sweeteners like maple syrup or honey instead of pancake syrup (especially the low-calorie ones). Also, try seasoning your food with fresh herbs and spices instead of pre-made seasoning blends or marinades.