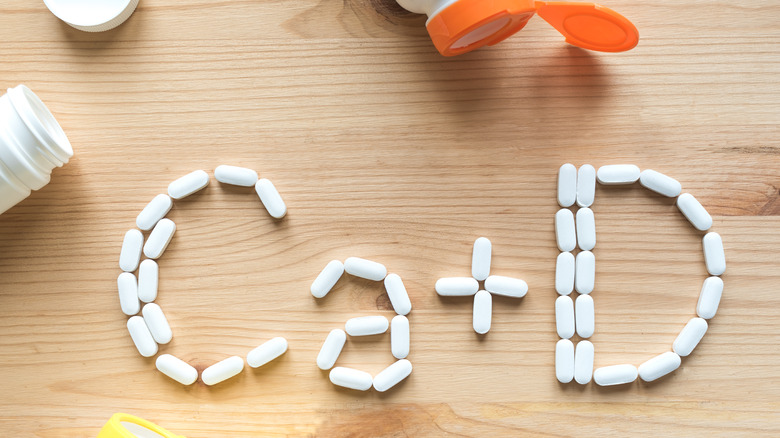Addison's Disease Explained: Causes, Symptoms, And Treatments
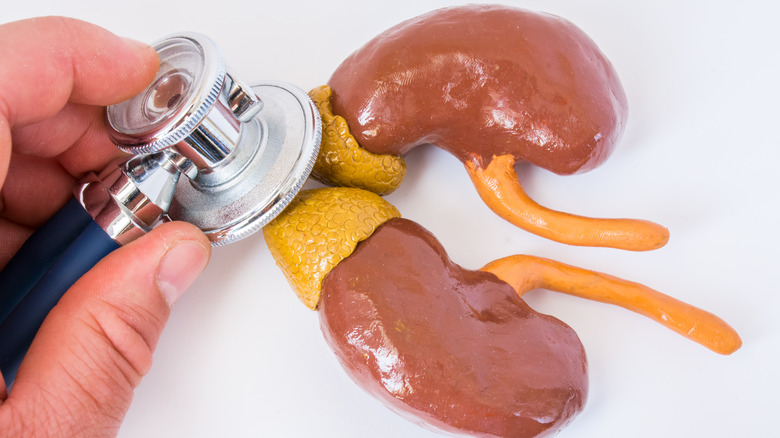
Addison's disease is an autoimmune disease characterized by insufficient production of the hormones cortisol and aldosterone by the adrenal glands, which are situated above one of two kidneys (via the Cleveland Clinic). Cortisol is an essential adrenal hormone that helps regulate blood pressure, heart rate, immune responses, and blood sugar levels. Through these physiological functions, cortisol mediates the body's stress response. Aldosterone, another adrenal hormone, maintains the body's balance of water and electrolytes (sodium and potassium), and thus plays a key role in keeping blood pressure stable.
According to a 2021 study published in the Journal of Clinical Pathology, Addison's disease is a rare disorder caused primarily by autoimmune destruction of the adrenal cortex (the outer part of the adrenal gland). There is often a delay in the diagnosis of the disease because subtle and non-specific symptoms that develop gradually over weeks and months are not easily recognized. Unfortunately, life-threatening adrenal crisis may be the first sign of Addison's disease. While there is no cure for Addison's disease, hormone replacement therapy is used to manage the condition.
The prevalence of Addison's disease in Europe has doubled since the 1960s to about 100-140 cases per million at the beginning of this century. These numbers continue to rise, likely because of better diagnostics and increased physician awareness. Striking women more often than men, Addison's disease primarily affects the 30 to 50 age group, though it can occur at any age.
Primary adrenal insufficiency
There are two main types of adrenal insufficiency: primary and secondary (per the Merck Manual). Addison's disease refers to primary adrenal insufficiency, which is most often caused by an autoimmune attack against the adrenal cortex. This results in damage to the cortex and inadequate secretion of the adrenocortical hormones cortisol and aldosterone (via StatPearls).
Failure of the adrenal glands to produce enough hormones can also be caused by infections such as tuberculosis, HIV, cytomegalovirus, and syphilis. Bleeding into the adrenal glands due to trauma, abnormal blood clotting, or tumors can impair adrenal function as well. In addition, certain drugs such as ketoconazole (antifungal) and etomidate (general anesthetic) can inhibit cortisol synthesis. Some conditions can damage the adrenal glands via infiltration of toxic substances such as excessive iron (hemochromatosis), abnormal proteins (amyloidosis), and cancer cells (metastatic cancer).
Other disorders that can lead to primary adrenal insufficiency include lymphoma, sarcoidosis, and disorders present from birth such as congenital adrenal hyperplasia.
Secondary adrenal insufficiency
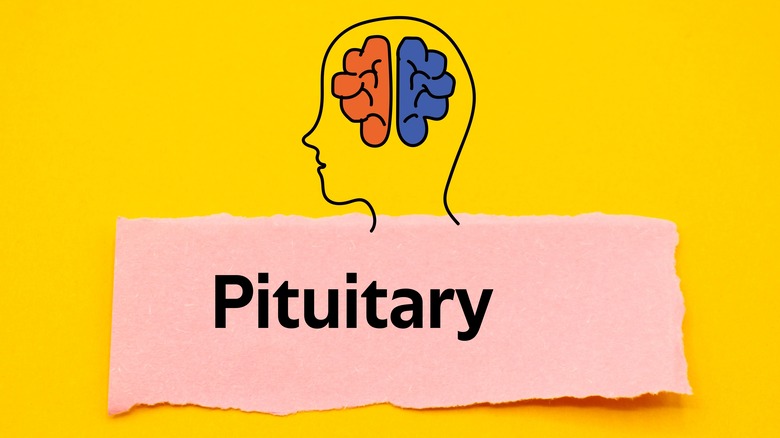
Although sometimes considered a form of Addison's disease, secondary adrenal insufficiency is a related disorder with similar symptoms but different causes, reports the Mayo Clinic. Normally, the pituitary gland produces a hormone called ACTH (adrenocorticotropic hormone) that signals the adrenal cortex to release cortisol and other hormones. In the case of secondary adrenal insufficiency, the pituitary gland makes less ACTH; consequently, hormone production (primarily cortisol) by the adrenal cortex is reduced.
Causes of secondary adrenal insufficiency include pituitary tumors, infection, and bleeding in the pituitary gland, notes Medical News Today. Genetic disorders involving the pituitary gland can also lead to reduced ACTH production while surgical removal of the pituitary would stop it completely. According to the National Adrenal Diseases Foundation, chronic use of prednisone and other glucocorticoid medications that function like cortisol can cause secondary adrenal insufficiency as well. Since the presence of prednisone in the body is perceived as cortisol by the pituitary gland, production of ACTH by the pituitary is scaled down.
If prednisone is stopped abruptly, temporary adrenal insufficiency may ensue because restoration of normal adrenal function is delayed. Therefore, it's imperative that the dose of glucocorticoids such as prednisone be tapered off over weeks to months.
Development of autoimmune Addison's disease
As noted in a review of studies published in a 2016 edition of the journal of Academic and Forensic Pathology, the most common cause of Addison's disease in developed countries is autoimmunity, coming in at 70-90%. This autoimmune condition is also referred to as autoimmune adrenalitis. The term aptly describes a process whereby chronic inflammatory cells infiltrate and damage the adrenal cortex, resulting in decreased production of adrenal hormones.
Autoimmunity in Addison's disease is triggered by an unknown event (possibly environmental) in genetically predisposed individuals, explains a 2014 article published in American Family Physician. The subsequent production of autoantibodies against 21-hydroxylase — an enzyme involved in the synthesis of cortisol and aldosterone (via MedlinePlus) — is an early sign of Addison's disease. In 90% of cases, these antibodies are formed long before the onset of overt disease.
The progression from the appearance of autoantibodies to clinical Addison's disease can take years to decades. As the disease progresses, elevated plasma renin is an early marker of adrenal deterioration, followed by loss of adrenal function and cortisol deficiency.
Risk factors for Addison's disease
A major risk factor for Addison's disease is a rare, genetic condition called autoimmune polyendocrine syndrome, reports the Cleveland Clinic. In this syndrome, multiple tissues and organs are erroneously perceived as foreign and subsequently attacked and destroyed by the immune system. Adrenal insufficiency is a hallmark characteristic of this condition (via the NIH).
Other autoimmune diseases also raise the risk of autoimmune Addison's disease (AAD), according to the Cleveland Clinic. Included among these are type 1 diabetes, pernicious anemia, Graves' disease, chronic thyroiditis, vitiligo, myasthenia gravis, and dermatitis herpetiformis, a skin rash associated with celiac disease. As noted by Healthline, people with cancer or chronic infections (e.g., tuberculosis), as well as individuals who have had partial surgical removal of the adrenal glands or are taking blood thinning medications, are also at high risk of AAD.
A 2019 review of studies published in the journal Therapeutic Advances in Endocrinology and Metabolism describes specific risk factors for life-threatening adrenal crisis, defined as a medical emergency featuring low blood pressure, abdominal symptoms, and abnormal laboratory findings that require emergency treatment. While intestinal infections and fever are the most frequent factors prompting adrenal crisis, other risk factors include stressful events (e.g., trauma, surgery, dental procedures), forgetting or discontinuing glucocorticoid (e.g., prednisone) therapy, major psychological distress, and exceptional physical activity.
Signs and symptoms of Addison's disease
As described by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), symptoms of Addison's disease progress slowly. Additionally, some of them are shared by other conditions, making the disease difficult to diagnose. Since the onset of symptoms can occur during potentially fatal adrenal crisis, early detection of signs and symptoms followed by treatment is critical.
People with Addison's disease predominantly experience long-lasting fatigue, muscle weakness, loss of appetite, abdominal pain, and weight loss. Per WebMD, other symptoms include nausea, vomiting, diarrhea, moodiness, irritability, depression, heat or cold intolerance, salt cravings, and poor stress management. Some people have low blood sugar while others experience low blood pressure when standing (postural hypotension), potentially causing dizziness or fainting.
Darkening or freckling of the skin, particularly visible in sun exposed skin, is also seen in people with Addison's disease. This blotchy, darkened skin has a greater tendency to occur on the forehead, knees, and elbows, as well as on scars, gums, skin folds, and creases (e.g., palms).
Addisonian crisis
During periods of physical stress, the adrenal glands respond by flooding the body with cortisol — often referred to as the 'stress hormone' — in amounts up to three times the normal amount, explains the Mayo Clinic. People with untreated Addison's disease are deficient in cortisol under ordinary conditions. When they are faced with physically stressful conditions (e.g., injury, infection, or illness) that require the production of higher levels of cortisol, their adrenal glands are unable to meet the challenge. A dangerous situation can ensue in which blood pressure, blood sugar, and blood potassium all drop. This life-threatening emergency is known as an Addisonian crisis (also called adrenal crisis) and requires immediate medical attention.
In 25% of people with Addison's disease, according to WebMD, an Addisonian crisis is the first manifestation of the disease. Other symptoms of Addisonian crisis include fever, weakness, fatigue, and sharp pain in the lower back, abdomen, or legs. Some people experience dehydration resulting from severe vomiting and diarrhea. A sudden drop in blood flow through the body can cause shock and loss of consciousness. Reduced blow can also lead to multiple organ failure, including the kidneys.
Diagnosing Addison's disease
As described in a 2014 article published in American Family Physician, the diagnosis of Addison's disease is difficult because symptoms are often vague (nonspecific) and usually come on gradually over time. A telltale sign is hyperpigmentation of the skin — the major distinctive feature of Addison disease (but not secondary adrenal insufficiency).
Abnormalities from routine blood tests (e.g., annual medical check-up) often alert physicians of a potential case of Addison's disease (via WebMD). These include low levels of sodium and iron (anemia) and high levels of potassium and eosinophils (immune cells). The confirmatory test for Addison's disease is the ACTH stimulation test which evaluates the capacity of the adrenal glands to make cortisol. A baseline blood level of cortisol is measured and then compared to cortisol levels measured after an injection of synthetic ACTH, a hormone that signals the release of cortisol from the adrenal glands. A response with low cortisol coupled with high ACTH is diagnostic of adrenal insufficiency (per American Family Physician). Measurement of antibodies against the enzyme 21-hydroxylase (essential for cortisol synthesis) can help determine whether the disease is due to autoimmunity.
Computed tomography (CT scan) may also be used to help identify the cause of Addison's disease. A CT scan can detect abnormalities such as small adrenals, bleeding, calcification related to tuberculosis, or masses.
How is Addison's disease treated?
According to a 2021 study in the Journal of Clinical Pathology, treating Addison's disease requires lifelong steroid hormone replacement. To replace cortisol, oral hydrocortisone is the first-choice therapy, followed by cortisone acetate. Compared to taking hydrocortisone in multiple doses throughout the day, a controlled release formulation taken once daily is better able to mimic the circadian pattern of circulating cortisol. To replace aldosterone, a once daily dose of fludrocortisone is taken to regulate sodium and fluids in the body. In addition to a deficiency of cortisol and aldosterone, a shortage of androgens is also common in people with Addison's disease. A 6-month trial of the androgen DHEA (dehydroepiandrosterone) is suggested in cases of persistent low energy levels, poor overall health, and poor libido (in women).
Side effects of hydrocortisone and fludrocortisone include insomnia, acne, delayed wound healing, dizziness, nausea, and excessive perspiration (via Medical News Today). Similar symptoms, as well as menstrual issues, facial hair, and a deeper voice, can occur in women taking DHEA.
To avoid an Addisonian crisis, the dose of hydrocortisone should be temporarily increased during stressful situations such as fever, infection, or other illnesses (via the NIH). This dose adjustment is indicated because the adrenal glands in people with Addison's disease cannot produce enough cortisol to meet the extra demands from added stress. Among people with Addison's disease, those who also have diabetes or are taking thyroid hormones, as well as pregnant women, may require dosage adjustments of steroid replacement therapy as well.
Ongoing management of Addison's disease
People with Addison's disease that are on steroid replacement therapy need to be closely monitored by their physician, reports the Mayo Clinic. Frequent contact with medical personnel is important to monitor signs of inadequate or excessive steroid replacement that require dosage adjustments. Moreover, an increased risk of type 2 diabetes, obesity, and osteoporosis is associated with excess hydrocortisone whereas high blood pressure can result from over-replacement therapy with fludrocortisone (via the Cleveland Clinic).
It is also advised to have a yearly checkup with a primary physician or endocrinologist, which may include screening for other autoimmune diseases, continues the Mayo Clinic. The reason for yearly screening is that people with one autoimmune disorder (e.g., Addison's) have an increased risk of getting another (via MedlinePlus). People with Addison's disease should consume plenty of salt, particularly during conditions that cause loss of salt from excessive perspiration (e.g., vigorous exercise and hot summer months). Salt can also be lost from bouts of diarrhea.
According to Medscape, people with Addison's disease should always wear an emergency medical alert bracelet to provide critical information to first responders. They should also be aware of the potential need to double or triple the dose of their steroid medications during stressful situations (e.g., illness, infection, or surgery). Instruction should be provided for the use of injectable hydrocortisone, which should be accessible when oral intake is not feasible due to, for example, excessive vomiting or diarrhea.
Diet/supplements and Addison's disease
Specific dietary changes may be beneficial in some people with Addison's disease, notes the NIDDK. For example, a high-sodium diet may be advised for some individuals with aldosterone deficiency, which causes urinary loss of sodium. Others may need to increase their consumption of calcium-rich dairy foods (or take calcium supplements) to protect against bone loss caused by high doses of corticosteroids.
In a 2021 clinical study in the European Journal of Endocrinology, a high prevalence of vitamin D deficiency was observed in European people with autoimmune Addison's disease (AAD). This finding is consistent with the established association of vitamin D deficiency with several autoimmune disorders. Vitamin D is critical for healthy immune function. A strong immune system is particularly important for people with AAD because they are susceptible to infections that can trigger an Addisonian crisis. They are also at high risk of developing other autoimmune disorders, cancer, and cardiovascular disease. Adequate vitamin D status in AAD is also important to ensure intestinal calcium resorption and reduce fracture risk. While genetics accounts for roughly 29% of vitamin D status, exposure to UV light is a likely contributing factor as well. Another contributing factor may be glucocorticoid (e.g., hydrocortisone) therapy, which can decrease intestinal absorption of vitamin D.
In another study in a 2018 edition of Nutrition, three months of high-dose vitamin D supplementation improved vitamin D status and was shown to regulate specific immune cells (T cells and monocytes) that play a role in the development of autoimmune disorders.
Prognosis
According to UpToDate, the prognosis of Addison's disease is good. With proper treatment and disease management, most people with Addison's disease can expect to live an active, normal life.
Per a 2021 study in the Journal of Clinical Pathology, treatment with glucocorticoids (e.g., hydrocortisone) saves lives and results in striking improvement in overall well-being. Nevertheless, people with Addison's disease must be on hormone replacement therapy for life. Even with medical improvements in recent years, they still have an increased risk of dying due in part to the inability to precisely duplicate the circadian rhythm of cortisol. An Addisonian crisis continues to be the first sign of the disease in many people; thus, medical personnel must be properly instructed to promptly recognize and diagnose the life-threatening condition.
Ongoing research is exploring methods to stop and possibly reverse the autoimmune deterioration of the adrenal cortex. One drug under study, rituximab, is an immunosuppressant that depletes B lymphocytes — immune cells that play an essential role in the development of autoimmunity (via the NIH). As reported by the Mayo Clinic, other potential future treatments for Addison's disease include pumps implanted beneath the skin that more accurately distribute steroid medications. Another promising option is the use of stem cells derived from the adrenal cortex, combined with immunotherapy, to modulate the immune response. Stem cells are also being investigated for their potential to regenerate adrenal cortex tissue in people with Addison's disease (per the Journal of Clinical Pathology).